Clinical evidence with Cellular Matrix®
Pain and mobility
CM-PRP-HA provided significant pain relief and functional improvement.1
A prospective multicenter study was performed in patients with knee joint OA who did not respond to treatment with HA alone. Patients received 3 injections of the RegenPRP-HA combination prepared with Cellular Matrix® (CM-PRP-HA).
- Number of patients: 77 (83 knees)
- Average age: 63
- Kellgren and Lawrence Grading Scale: grade II (43.4%) and grade III (56.6%)
Results
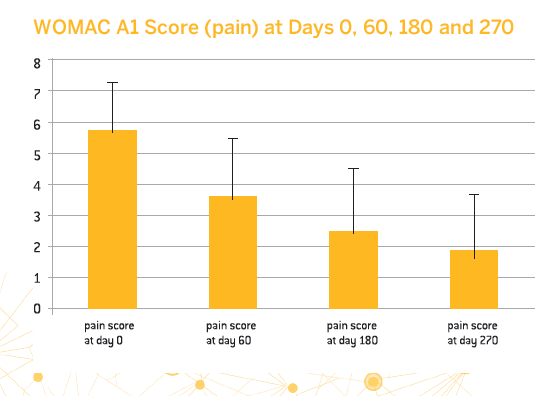
- 83.6% of patients were strict responders to treatment with CM-PRP-HA based on the OMERACT-OARSI criteria
- A significant decrease in pain as evaluated by the WOMAC A1 score was observed between day 0 and day 270
- The treatment provided long-lasting benefits for half of the patients and allowed almost 80% of them to avoid surgery for at least 4 years
Conclusion
This study demonstrated that 3 injections of CM-PRP-HA is a new medical approach for patients who do not respond to treatment with HA alone and offers an alternative to knee surgery.1
83.6% success rate in patients who had an unsatisfactory clinical response to previous therapy with HA alone.1
New therapeutic option
Cellular Matrix technology: a safe, fast and novel treatment approach for OA of the knee.2
Fifty-three patients (90 knees), aged 39-80 years, suffering from knee osteoarthritis (Kellgren-Lawrence Grade I-III) were enrolled in a prospective randomized controlled clinical study comparing the clinical efficacy of Cellular Matrix (CM-PRP-HA) with two different types of HA: a non-crosslinked sodium hyaluronate (AV) and a non-crosslinked sodium hyaluronate combined with mannitol (OP).
- CM-PRP-HA group (19 patients, 30 knees): 3 injections (3 mL PRP + 2 mL HA) every 15 days,
- AV group (19 patients, 30 knees): a weekly injection of HA (2 mL) for a total of 3 weeks,
- OP group (15 patients, 30 knees): a weekly injection of HA + mannitol (2 mL) for a total of 3 weeks.
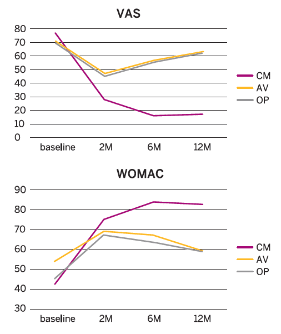
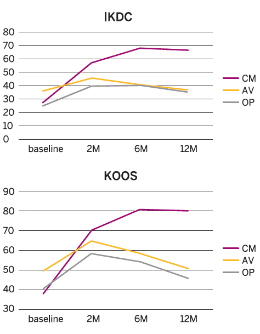
Results
- Pain and functional outcomes, assessed using the VAS, WOMAC, KOOS and IKDC scores, showed a statistically significant improvement at 2, 6 and 12 months after the last injection compared to baseline values.
- The improvement in the CM-PRP-HA group was significantly better compared to the other two groups at 2 months and highly significant (p<0.01) at 6 months and 12 months.
- Statistically significant improvement of the cartilage thickness was observed at 2, 6 and 12 months after the end of CM-PRP-HA treatment on both lateral (p<0.05), and medial (p<0.01) compartments.
Conclusion
The Cellular Matrix PRP-HA combination results in a longer and sustained clinical improvement than HA alone, and thus might be a useful tool for postponing arthroplasty.1,2
Bibliography
- Renevier, J. L., et al. «Cellular matrix™ PRP-HA”: A new treatment option with platelet-rich plasma and hyaluronic acid for patients with osteoarthritis having had an unsatisfactory clinical response to hyaluronic acid alone: Results of a pilot, multicenter French study with long-term follow-up.» Int. J. Clin. Rheumatol. 2018;13(4):230-8
- Barac, B., et al. (The new treatment approach in knee osteoarthritis: Efficacy of cellular matrix combination of platelet rich plasma with hyaluronic acid versus two different types of hyaluronic acid (HA). Int. J. Clin. Rheumatol. 2018;13(5):289-95
Our products
Clinical evidence with Regenkit A-PRP
RegenPRP in OA
Objective:
Prospective, randomized, dose-controlled, placebo-controlled, double-blind, triple-parallel study to compare the efficacy of intra-articular injections of RegenPRP or Hyaluronic Acid (HA) or normal saline (NS) solution for knee OA1
Number of patients treated and characteristics:
53 patients diagnosed with osteoarthritis, Ahlbäck Grade I-III, were randomly assigned to 3 groups.
Treatment:
3 weekly injections of either RegenPRP (31 knees), HA (29 knees), or NS (27 knees).
Follow-up: WOMAC and IKDC scores were recorded at 1, 2, 6 and 12 months
Results
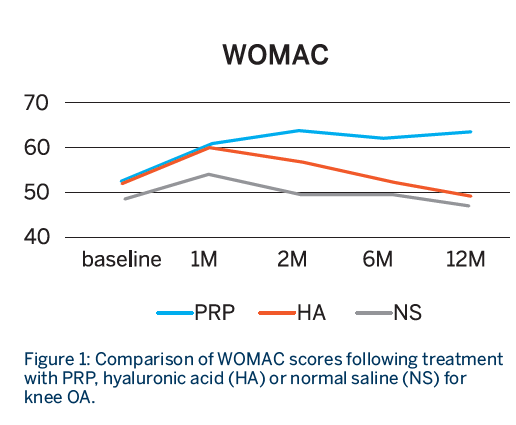
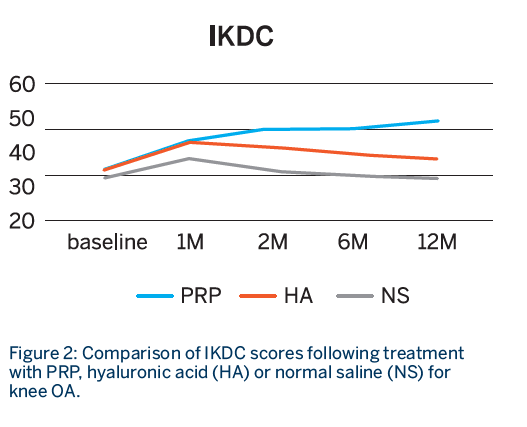
- All groups showed statistically significant improvement in both scores at 1 month.
- Sustained improvement up to 12 months for both WOMAC and IKDC scores was observed only in the PRP group, whereas both the HA and NS groups showed declining scores after the first month.
- The improvement was only sustained in the PRP group at 12 months.1
RegenPRP on the quality-of-life
Significant improvement in function and pain at all time points compared to baseline.
Objective:
Evaluate and quantify the effects of RegenPRP on the Quality-Of-Life (QoL) of knee OA patients2
Number of patients treated and characteristics:
72 patients with knee OA, Kellgren-Lawrence Grade 2 or 3
Protocol:
Patients had a total of 3 treatment sessions, with a RegenPRP injection given every 21 days. Patients were evaluated before the treatment and at 1, 3, 6 and 12 months from the last injection using the WOMAC, VAS at rest and VAS in movement scores.
Results
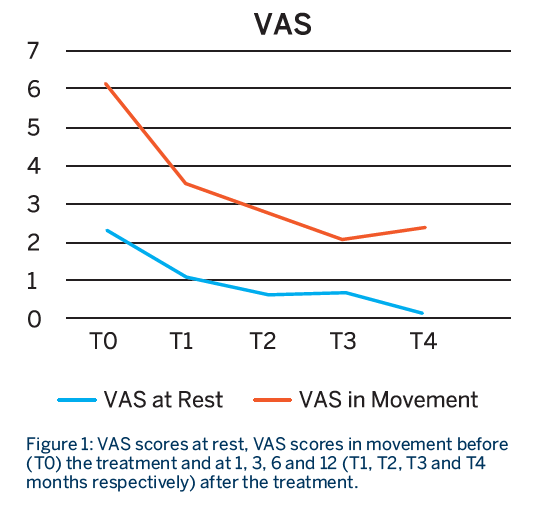
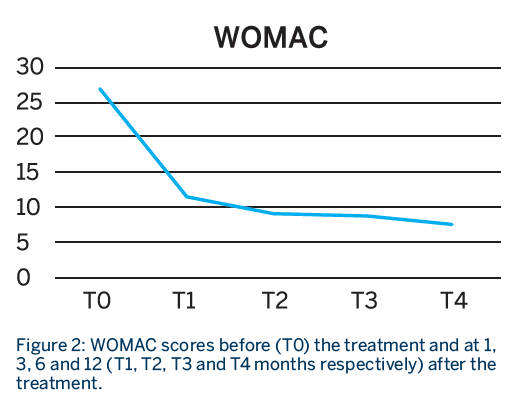
- Patients showed a significant improvement in function and pain at all time points compared to baseline, which lasted for almost one year.
- A breakdown analysis of the items on the WOMAC score indicated that there was a statistical improvement in the pain and function score until the third month, after which it did not improve further but was sustained.
Bibliography
- Lin, K.-Y., et al. (2019). «Intra-articular injection of platelet-rich plasma is superior to hyaluronic acid or saline solution in the treatment of mild to moderate knee osteoarthritis: a randomized, double-blind, triple-parallel, placebo-controlled clinical trial.» Arthroscopy 35(1): 10100106-117.
- Mangone, G., et al. (2014). «Infiltrative treatment with Platelet Rich Plasma (PRP) in gonarthrosis.» Clin Cases Miner Bone Metab 11(1): 67-72.
Contact Regenlab
Now is the time to change your patient’s life. Browse ressources and
get connected to treatment that is right for them.
For more information on our products, please contact us by clicking on the button below:
Our Regional offices are
located in
- New York (USA)
- Montréal (Canada)
- Venice (Italy)
- Munich (Germany)
- Paris (France)
- Dubai (U.A.E.)
- Beijing (China)
- Istanbul (Turkey)




