Clinical evidence with Regenkit® A-PRP technology
There is growing interest in the use of several innovative technologies being routinely used for facial skin care and skin rejuvenation for the treatment of genital skin disorders resulting from the aging process or with inflammation-related origins. This includes the use of PRP.
Lichen sclerosus
Lichen sclerosus (LS) is a chronic inflammatory dermatitis that creates patchy, white skin that appears thinner than normal. It usually affects the genital and anal areas. Most symptomatic patients complain of pruritus and burning or soreness, dysuria or dyspareunia.
Clinical study description:
In a prospective study3, 40 women with lichen sclerosus and papillomavirus infection of the vulva were injected with 4- 8 ml of RegenPRP® in the affected area using a mesotherapy technique. The next day, antiviral Panavir gel was applied to the lesion followed by low-intensity laser light treatment.
- Patients received 3 courses at 4-week intervals. Severity of nine symptoms were evaluated, among them itching, hypopigmentation, and oedema.
Results
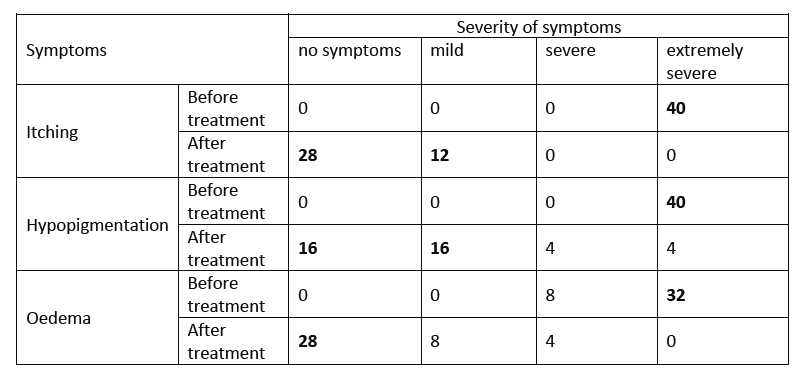
- Before treatment, all 40 patients presented with extremely severe symptoms of itching and hypopigmentation; in addition, 32/40 patients presented with extremely severe symptoms of oedema (See Table).
- One month after treatment, 12/40 patients reported only mild itching, the remaining 28 patients were without itching symptoms. The number of patients reporting severe hypopigmentation was reduced to 4, with 32 patients reporting no or mild hypopigmentation.
- Twenty-eight patients reported no more oedema symptoms (See Table).
Conclusion
- Overall, the treatment in patients with early signs of lichen sclerosus was effective in 90% of cases (36/40) as indicated by a significant decrease in all clinical symptoms that were evaluated.
SAFETY
- No complications were seen after the treatment.
Female sexual dysfunction
Female sexual dysfunction may be manifested by decreases in sexual desire, arousal and lubrication, leading to dyspareunia.
d
Clinical study description:
Eleven women (aged 24-64 years old) presenting with complaints of dyspareunia or other symptoms relating to sexual dysfunction were included in a case series.4
- RegenPRP® was activated prior to use by the addition of calcium solution and was injected within 10 minutes of activation into the anterior vaginal wall and the clitoris.
Results
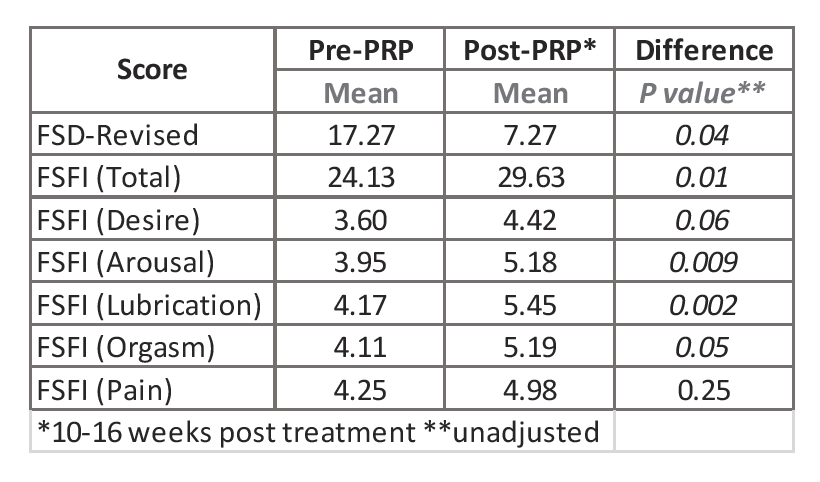
- Prior to treatment, seven of the patients had a Female Sexual Distress (FSD) score > 11.
- At 12-16 weeks post-injection, five of the women had a FSD score ≤ 7, and one patient had decreased her FSD score from 34 to 14.
- Evaluations based on the Female Sexual Function Index (FSFI) score indicated an overall significant improvement after injection. The scores from the subscales of desire, arousal, lubrication, and orgasm were all significantly increased after injection of RegenPRP®. There was no change in the scores for pain (See Table).
Wilcoxon signed rank sum test was used. P < 0.05 is significant.
Conclusion
- The results of this pilot study suggest that RegenPRP® injections could be effective for the treatment of certain types of female sexual dysfunction
SAFETY
- No adverse events were reported.
Our products
Clinical evidence with Regenkit® A-PRP technology
There is growing interest in the use of several innovative technologies being routinely used for facial skin care and skin rejuvenation for the treatment of genital skin disorders resulting from the aging process or with inflammation-related origins. This includes the use of PRP.
STRESS URINARY INCONTINENCE
STRESS URINARY INCONTINENCE
Clinical study description:
In a prospective intervention study, 20 women suffering from stress urinary incontinence (SUI) were treated with RegenPRP5.
- Patients received a monthly injection for 3 consecutive months.
- With a 27-gauge needle, RegenPRP® was injected into the anterior vaginal mucosa around the patient’s mid-urethra (2 ml), which was approximately 1 cm below the urethra meatus with a depth about 1.5 cm, and 1.5 mL was also injected on each side of the urethra.
Results
RegenPRP® was effective in relieving SUI symptoms at both 1 month and 6 months post-treatment:
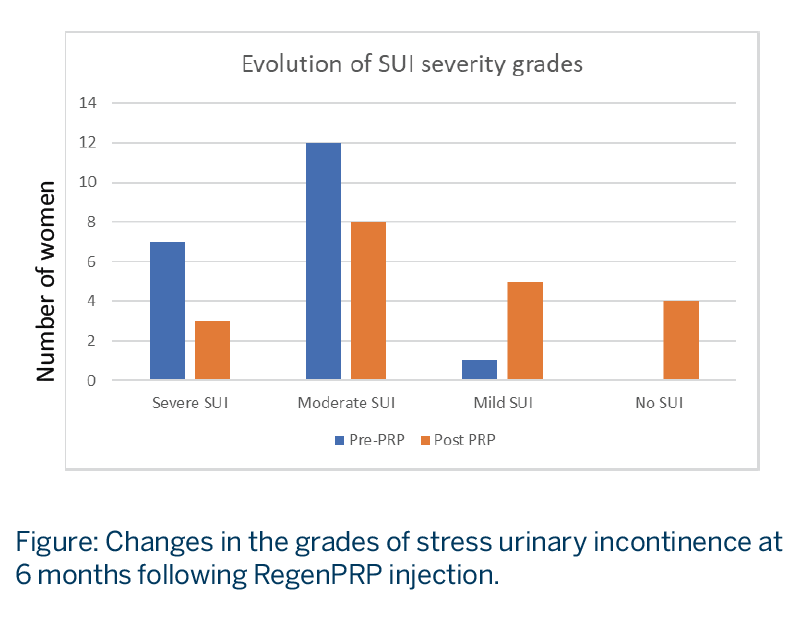
- SUI grade, evaluated with the International Consultation on Incontinence Questionnaire-Short Form (ICIQ-SF) score, illustrated a shift in the distribution of disease severity to milder disease after PRP treatment. See Figure.
- Treatment efficacy as assessed by ICIQ-SF, Urogenital Distress Inventory (UDI-6), Incontinence Impact Questionnaire (IIQ-7) and Overactive Bladder Symptom Scores (OABSS) showed a significant improvement in incontinence. All these scores revealed significant and lasting effectiveness.
- Younger women (<40 years old) tended to have better treatment outcomes (P = 0.07).
- The urodynamic studies performed on 8 women showed that residual urine and bladder volume at first sensation to void increased significantly 6 months after injection of RegenPRP.
improvement in symptoms.
Conclusion
- This pilot study is the first to report the effective treatment of SUI in women with PRP.
- Local injection of RegenPRP® seems safe with a satisfactory response in treating mild to moderate SUI in females both at 1 month and 6 months post treatment.
- This innovative intervention could be an alternative treatment for SUI, but further studies are needed.
SAFETY
- No adverse reactions were reported.
Bibliography
- Fountain, John H., and Sarah L. Lappin. Physiology, Platelet. StatPearlsPublishing, Treasure Island (FL), 2019.
- Mathew, Joscilin, and Matthew Varacallo. Physiology, Blood Plasma. StatPearls Publishing, Treasure Island (FL), 2019.
- Chernova, N. I., et al. (2016). “Experience in management of patients with lichen sclerosus and HPV infection of the vulva; Опыт ведения пациенток со склеротическим лихеном и папилломавирусной инфекцией вульвы.” Russian Journal of Clinical Dermatology and Venereology 15(5): 72-76.
- Runels, C., et al. (2014). “A Pilot Study of the Effect of Localized Injections of Autologous Platelet Rich Plasma (PRP) for the Treatment of Female Sexual Dysfunction.” J. Women’s Health Care 3(4).
- Long, C.-Y., et al. (2021). “A pilot study: effectiveness of local injection of autologous platelet-rich plasma in treating women with stress urinary incontinence.” Sci Rep 11(1): 1584.
Contact Regenlab
Now is the time to change your patient’s life. Browse ressources and
get connected to treatment that is right for them.
For more information on our products, please contact us by clicking on the button below:
Our Regional offices are
located in
- New York (USA)
- Montréal (Canada)
- Venice (Italy)
- Munich (Germany)
- Paris (France)
- Dubai (U.A.E.)
- Beijing (China)
- and Istanbul (Turkey)




