What is RegenKit® Extracell BMC ?
What
- The Flexible & Reproducible Bone Marrow Concentrate System
- Class IIb CE certified Medical Devices

Bone marrow aspirate vol per tube
5 to 10 ml

MNC recovery
> 70%

MSC recovery
> 80%
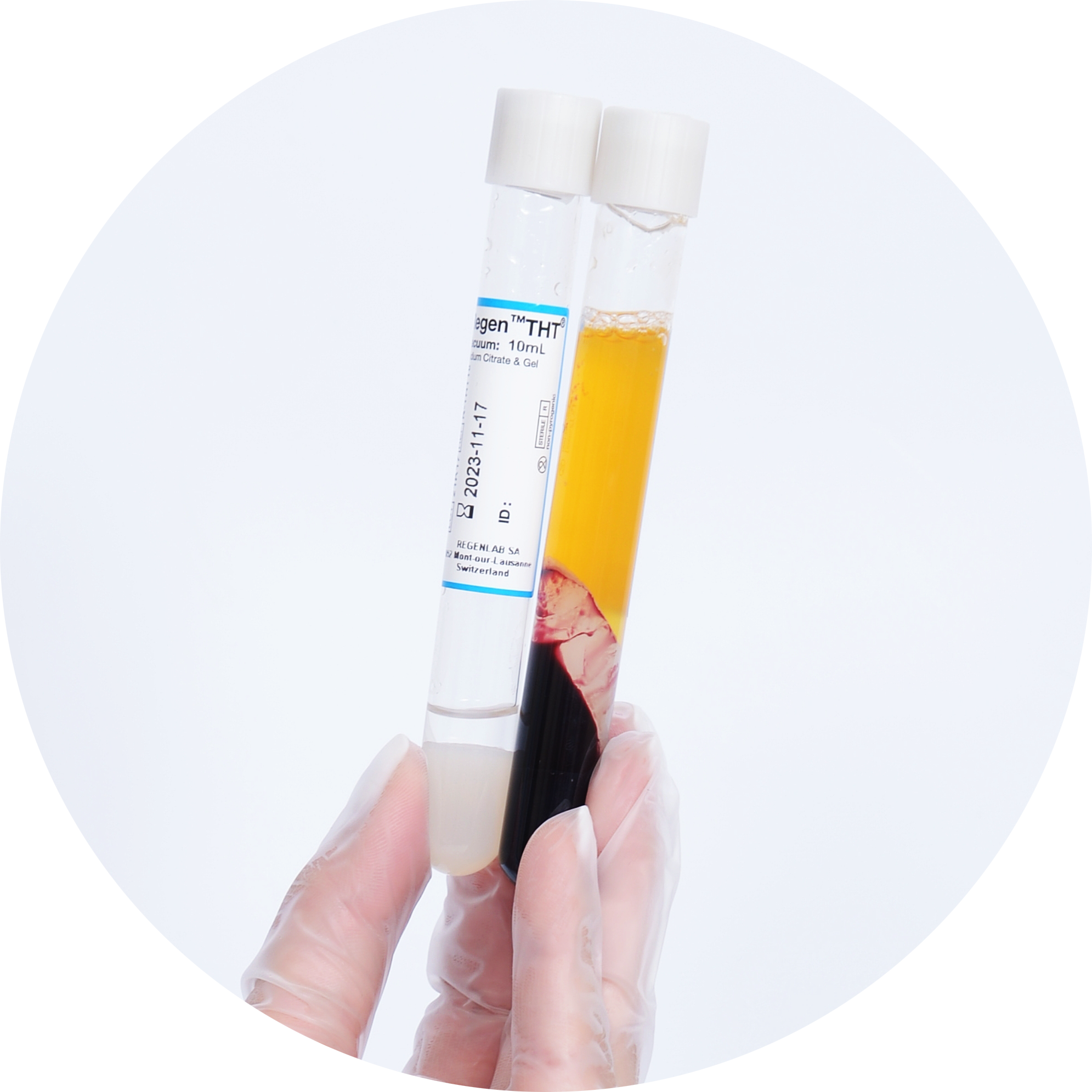
RegenTHT Tube Properties 1
Bone Marrow Concentrate preparation with RegenKit® Extracell BMC
Bone marrow concentrate (bmc) is an autologous cell concentrate from bone marrow aspirate.
Bone marrow is a liquid tissue rich in a heterogeneous population of mature cells and multipotent stem cells.
Among them, the mesenchymal stromal cells (MSC) are the musculoskeletal tissue precursor cells.
RegenKit® Extracell BMC kits are specifically designed for the effective processing of bone marrow aspirate (BMA) by a short centrifugation in RegenTHT tubes. These tubes contain a biologically inert separating gel with a specific density that physically separates the plasma and the mononuclear cell (MNC) fraction, which contains the MSC, from the other bone marrow cellular elements.
More than 80% of the MSC are recovered in the resulting cell concentrate1. In vitro, these cells give rise to fibroblast colony-forming units (CFU-F) capable of undergoing osteogenesis, chondrogenesis and adipogenesis when cultured in their respective specific induction medium1.
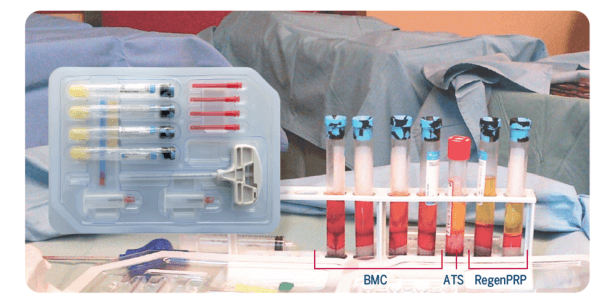
Example of a procedure: Bone marrow was processed with the four tubes of the RegenKit® Extracell BMC.
The resulting BMC can be combined with platelet-rich plasma (RegenPRP) and autologous thrombin serum (ATS), prepared with RegenKit® Surgery, to obtain a gel.
Our products
Intensed use of the device
Preparation of autologous bone marrow cell concentrate
Preparation of Autologous Platelet Rich Plasma & other plasma-derived products
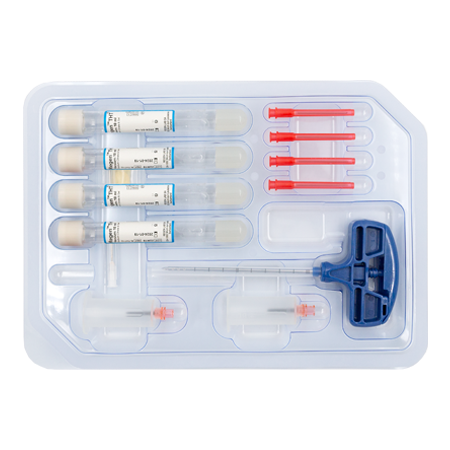

RegenKit® Extracell BMC
1 Trocar
4 RegenTHT tubes
2 Transfer devices
4 Transfer needles
1 x 80 mm transfer cannula
Self-adhesive discs
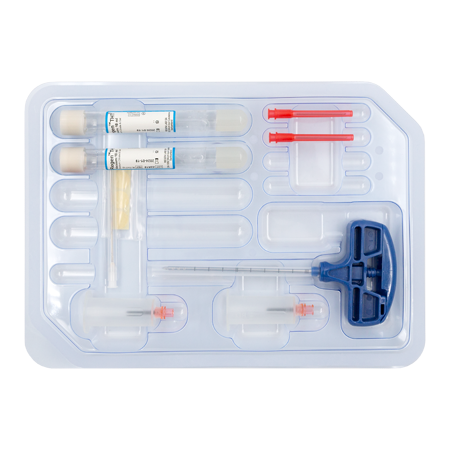

RegenKit® Extracell BMC 2
1 Trocar
2 RegenTHT tubes
2 Transfer devices
2 Transfer needles
1 x 80 mm transfer cannula
Self-adhesive discs
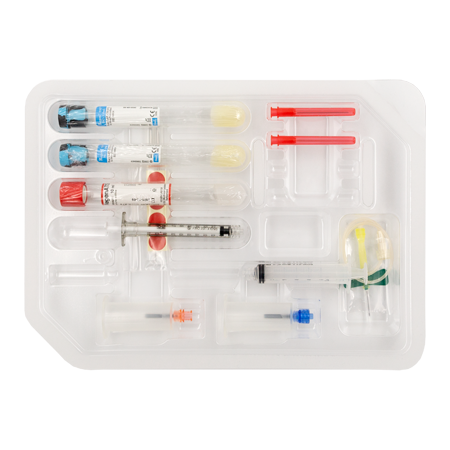

RegenKit® Surgery
1 Safety–LokTM Butterfly needle
1 Collection holder
2 RegenTHT tubes
1 RegenATS tube
1 x 1 ml Luer-LokTM syringe
1 x 5 ml Luer-LokTM syringe
1 Transfer device
2 Transfer needles
2 x 80 mm transfer cannulas
Self-adhesive discs
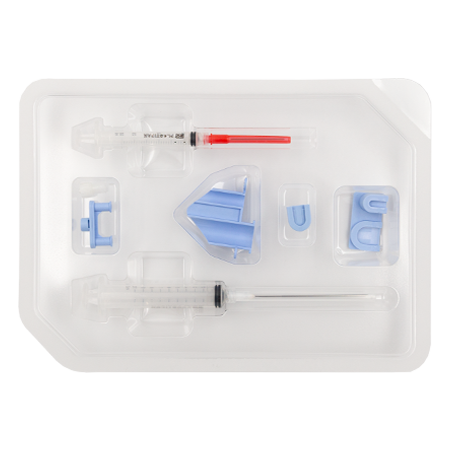

RegenSpray Applicator
RegenSpray Applicator
1 x 10 ml Luer-LokTM syringe
1 Nozzle for spray application
1 Double piston stopper
1 Applicator syringe holder
1 x 3 ml Luer-LokTM syringe
1 3:10 ratio adapter
1 Luer-lock connector (FM)
1 x 80 mm transfer cannula
1 Transfer needle
Class IIb CE certified Medical Devices. Regen Lab SA is an ISO 13485 :2016 and MDSAP certified medical device manufacturer
Warnings and precautions
This kit is intended only for bone reconstruction, orthopedic and traumatological surgeries.
A strict aseptic technique must be followed during bone marrow aspirate. Use proper safety precautions to avoid contact with patient blood or cross-contamination. Use proper safety precautions to guard against needles or broken tubes. Do not use sterile component of this kit if package is opened or damaged. Do not use components of this kit if they are broken or present a defect. Do not use the tube if it lost vacuum. Do not use the sodium citrate solution or other tube components alone. Store between 5ºC and 30ºC; bring kit to ambient temperature before using the tubes. Do not re-sterilise, do not use after the expiry date. Single use device, do not reuse any part of the kit. Reuse may lead to infection or other illness / injury. Transfer needle must be used to transfer liquids only and should not be used for injection. The preparation of the bone marrow concentrate must be performed by a physician trained on the equipment and procedure, or under the supervision of the physician. The treatment with bone marrow concentrate must be performed by a qualified physician. Do not inject bone marrow cell concentrate intravascularly. The patient must be informed of the general risks associated with the treatment and of the possible adverse effects. The safety and effectiveness of combination of bone marrow cell concentrate with other therapies should be assessed by the physician. The safety and effectiveness have not been evaluated in children and in pregnant or lactating women.
The bone marrow cell concentrate must be prepared from fresh blood and must be used within four hours (extemporaneous use only). All tubes and components of the kit are to be entirely discarded by elimination method after each use to avoid potential contamination with blood products. Use a 45° fixed angle rotor centrifuge or a horizontal head swinging bucket centrifuge (ex. RegenPRP Centri provided by Regen Lab). Follow the manufacturer’s instructions when using the centrifuge. Tubes should be centrifuged, as recommended in the instructions for use, at a relative centrifugation force (RCF) of 1500 g. Excessive RCF (over 2200 g) may lead to tube breakage resulting in blood exposure, and possible injury. RCF below 1500 g may lead to incorrect blood separation and red blood cell contamination of bone marrow cell concentrate. Centrifuge carriers and inserts size should be adapted to the tubes. Use of carriers too large or too small may result in breakage of the tubes. Caution should be taken to ensure that tubes are properly seated in the centrifuge carriers. Tubes must be balanced in the centrifuge.
Possible adverse effects
Haemorrhage, hematoma, delayed wound healing. Early or late operative infection. Persistent pain, seromas. Allergic reaction to anaesthesia.
Patented by Regen Lab SA
Platelet Rich Plasma
U.S. patent US8529957, US11241458, US11110128, US11096966, US10881691,
US10092598, US10080770, US10064894, US8529957, US9833478, US10052349, US11241458
European patent EP2073862B, EP3111974B1
Swiss patent CH696752
Hong Kong patent HK1231793
Bibliography
- Barry, F., A Report on The Phenotypic Characterisation of Mesenchymal Stem Cells isolated from Human Bone Marrow Using the RegenTHT, A-CP and Regen BCT Blood Separation Systems. 2015, The Regenerative Medicine Institute (REMEDI), National University of Ireland Galway.
Contact Regenlab
Now is the time to change your life. Browse resources and get
connected to treatment that is right for them.
For more information on our products, please contact us by clicking on the button below:
Our Regional offices are
located in
- New York (USA)
- Montréal (Canada)
- Venice (Italy)
- Munich (Germany)
- Paris (France)
- Dubai (U.A.E.)
- Beijing (China)
- Istanbul (Turkey)




